 Part 1. Diagnostic Overview
Part 1. Diagnostic Overview
Summary
Bovine trichomoniasis is a disease of the reproductive tract caused by Tritrichomonas foetus, a flagellate protozoan. In cows, infection leads to embryonic and early foetal death, abortion, foetal maceration, pyometra and transient or permanent infertility. Asymptomatic infection occurs in bulls, which become persistent carriers and the main reservoir of infection. Young bulls are less susceptible to infection. Heifers have a higher incidence of the disease compared to cows, as the latter may remain immune for up to three years post-infection.
The disease is found in all cattle-producing countries in the world. In some parts it still causes major economic loss due to abortion, infertility and the culling of carrier bulls. The disease is found in extensively farmed beef cattle, but in intensively farmed beef and dairy cattle the use of artificial insemination has reduced the incidence of the disease. As a result, it is seldom seen in cattle in New Zealand or southern parts of Australia, but it still occurs in northern Australia.
Bovine trichomoniasis is diagnosed by detection of the parasite in direct smears or culture, or by PCR of preputial washings, vaginal material or aborted tissues. Other trichomonads may be present in samples and need to be differentiated from T. foetus through either morphology or PCR.
The disease has occurred in Australia since 1946 and in New Zealand since 1937. Cattle and buffalo semen imported into Australia must be free of T. foetus.
Breeding centres must demonstrate that bulls are free of T. foetus.
The OIE states that for international trade requirements detection of T. foetus by either direct examination or culture is the current prescribed test. Although PCRs are available, further validation is needed before they can be accepted as a prescribed test.
Aetiology
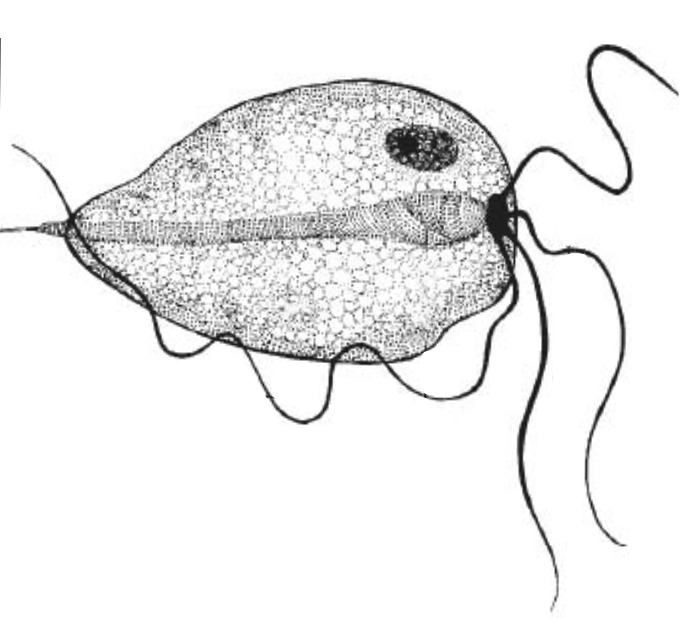
The causative agent of bovine trichomoniasis is Tritrichomonas foetus (Riedmüller, 1928), which belongs to the Class Parabasalia, from the classification system of Dyer, where protozoa with the “9+2” flagellum belong to the phylum Zoomastigina. The protozoan is 8-18 µm long, 4-9 µm wide, pyriform (pear-shaped) but with considerable pleomorphism. It is characterised by three anterior flagella of length 11-17 µm and one posterior flagellum 16 µm in length. An undulating membrane with 2-5 waves is positioned lengthways along the organism and extends to form the posterior flagellum. The axostyle is prominent with a chromatic ring at the point of emergence from the posterior end of the organism.
T. foetus consists of only one trophozoitic form and has a simple lifestyle. The trophozoites can adopt a spherical shape, known as a pseudocyst, and internalise (or retract) their flagella. The formation of pseudocysts occurs during times of stress either inside the body of the host or in culture media and also during adhesion to and subsequent phagocytosis of sperm cells. The role of the pseudocyst form is not fully known but it may be involved in transfer to a new host or attachment to host cells. Pseudocysts can generate multinucleated organisms that, under favourable environmental conditions, release single organisms. Pseudocysts also occur in preputial samples at a rate of 55% compared to pear-shaped parasites at 20%. T. foetus does not form cysts and cannot survive outside the host.
Three serotypes of T. foetus have been described worldwide and all have been reported in Australia: var brisbane, var belfast and var manley, with the former two being the most common in Australia.
T. foetus is indistinguishable from T. suis (found in nose and gut of pigs) according to morphology, pathogenic potential in subcutaneous mouse assays, by DNA fingerprinting using restriction fragment length polymorphism and variable-length DNA repeats, and by comparison of the 16S rRNA gene.9 Serological properties shared by the two organisms also suggest they are the same species. T. foetus causes chronic large bowel diarrhoea in cats; however, sequence information from the TR7/TR8 variable-length repeat within the internal transcribed spacer region and within the elongation factor-1 alpha (EF-1) gene12 suggests a bovine genotype and a feline genotype.
Other trichomonads may be present in the bovine genital tract: Pentatrichomonas hominis, Tetratrichomonas buttreyi, Tetratrichomonas pavlova, Tritrichomonas enteris and Pseudotrichomonas species. Trichomonas tenax occurs infrequently in humans in the oral gingival or in tracheobronchial sites, and P. hominis occurs infrequently in the intestinal tract. These species only transiently infect heifers.15 The organisms do not persist in the reproductive tract, specific antibodies of IgA type is not detected, and only a mild inflammatory response is produced compared to infection with T. foetus.
Mechanisms leading to infertility and abortion are related to the parasite’s ability to infect the mucosal surfaces of the reproductive tract, to bind to spermatozoa and to release a cysteine protease that induces cell death. Adherence of T. foetus to spermatozoa results in loss of motility, agglutination and release of lysozymes that digest the sperm. In the cow, T. foetus moves through the reproductive tract where it adheres to and infects vaginal epithelial cells, uterine epithelial cells, oviduct cells, and in pregnant cows, the placenta. Adherent T. foetus cells release a cysteine protease (CP30) that produces cytopathic effects in oviduct cells and in epithelial cells of the vagina and uterus resulting in apoptosis. The cysteine protease is capable of cleaving IgG2 and evading the host immune response.
Clinical Signs
In trichomoniasis, overt clinical signs are not seen. Evidence of the disease in a herd is chronic infertility, returns to service after four to five months (with a higher incidence in heifers compared to cows), aborted foetuses (at any time during gestation from two-months onwards but more commonly from three to five months), and persistent vaginal discharge post coitus.
In cows, clinical signs vary from mild vaginitis or endometritis, to acute inflammation of the entire reproductive tract. Infection during pregnancy leads to early embryonal death, abortion and, sporadically, pyometra.
Return to service within three to five weeks post-coitus indicates infection and related failure to become pregnant, or early termination. Reduced calving rates of approximately 18% are greatest in the first two years in cows experiencing infection for the first time, with decreasing production loss in subsequent years.
Cows recovering from infection are generally resistant to the infection for one to three years although this varies between animals.
No clinical signs are present in infected bulls.
Epidemiology
The major route of transmission of T. foetus is through coital contact between a bull and a heifer or cow, although contaminated artificial insemination (AI) equipment may also transmit the infection.
An infected bull is the main reservoir of infection and is a major risk factor for ongoing disease in a herd. The infective dose for three- to seven-year old bulls is 106 organisms, but some can be infected with as little as 102 organisms, indicating a likely difference in host susceptibility. Older bulls (four, five and six years of age) are more susceptible to infection when naturally mated to infected heifers, whereas younger bulls (three year olds) are less susceptible. The exclusive use of young bulls may reduce the incidence of the disease in cows in extensively managed herds.
The infective dose for cows is usually 103 organisms.22 Infection in previously non-infected cows is most likely to occur when the time interval between services is less than 20 minutes. Once cows are infected, T. foetus can be isolated two weeks after mating. Passive transmission (transmission of the organism from infected to non-infected cows via a non-infected bull) can occur but the transmission rate is low.
Survival of T. foetus in the uterus occurs for up to 22 months. It causes inflammation, which either prevents conception, or leads to abortion. Cows may recover from infection, usually within 90 days and over a number of oestral cycles, but reinfection is common. IgG1 opsonic antibodies are produced in the vaginal mucus and antibodies of class IgG2 are produced in the serum and may result in immunity for up to nine months post-infection, but there is a progressive loss of immunity over the following 20 months during which the animal can become re-infected. Immunity does not develop with age.
The immune response in infected bulls is poor, and consistent with a carrier status. Agglutinating antibodies are not detected in the preputial cavity.26 The immunological response is not reliable for diagnostic purposes, hence serology is limited in its use for detection of T. foetus, due to poor sensitivity and specificity. The antibody titre in serum may be 1:32 without infection but increases to 1:512 in heifers 11 weeks post-infection, and to 1:128 in bulls. Non-specific agglutinins against T. foetus have been reported from a variety of animals including frogs, birds, rabbit and horse.
Immunisation with a vaccine containing killed cells of T. foetus prevents genital infection in most bulls up to the age of five years; however, it does not effectively prevent or cure infection in bulls older than five and a half years.27 Vaccinated bulls challenged with T. foetus produce a systemic and genital immune response due to IgG1 and IgG2 antibodies, but unvaccinated bulls, when challenged, do not produce an antibody response and persistent infection occurs.28 Vaccination reduces the rate of abortion by only 30%.
Serotyping of the protozoan is no longer performed, but all three serotypes of T. foetus have been reported in Australia. In north-eastern Australia a study found a distribution of 80% for T. foetus var brisbane and 20% for T. foetus var belfast. In NSW var belfast predominated with a low incidence of var manley. Serotypes var belfast and var brisbane were the only serotypes detected in WA.
The host range for T. foetus includes cattle (Bos taurus and B. primigenius indicus), horses, roe deer, cats and pigs. Other mammals such as domestic cats, horses and roe deer can be hosts to T. foetus and experimental infection has been established in rabbits, golden hamsters, guinea pigs, dogs, pigs and goats.
Occurrence and Distribution
Bovine trichomoniasis was first reported in France in 1888. It has a worldwide distribution especially in countries where natural service is commonly practised. It has been reported from Argentina, Austria, Canada, Czech Republic, Denmark, Germany, Hungary, Italy, Japan, Kenya, Mexico, Norway, Serbia and Montenegro, Slovakia, Spain, South Africa, Switzerland, Poland, Puerto Rico, Romania, Russia, United Kingdom and United States, although nowadays, with the use of AI, the disease is seen rarely in many countries.
In Australia, bovine trichomoniasis was reported in 194835 to have occurred on King Island in 1946 and in Queensland in 1950. It is widespread in extensively managed beef herds in the northern parts of Australia. In the Northern Territory, the disease occurs in the districts of Darwin, Victoria River, Elsey, Gulf and Barkly Tablelands, with a low incidence around the Alice Springs district. The last survey, in the mid-eighties, reported 65.6% of herds infected (1,008 bulls were tested on 41 stations in the Victoria River district), with a prevalence in infected herds of 2.9-33.3% (an average of 11.7%). In Queensland, a 1974 study indicated 17.5% of herds were infected.31 In Western Australia (WA), a 1977 survey of T. foetus through abattoir sampling of bull genitalia indicated a prevalence of 1.8% in pastoral areas and 28.2% in the Kimberley region. T. foetus was not found in samples from the south-west of WA.32 The disease is rarely seen in intensively managed herds in the southern parts of Australia due to the use of AI.
In New Zealand the disease was first reported in 1937.39 It has been reported infrequently since then, from a beef herd in the Tokomaru Bay area in 198240 and from the Hawke’s Bay/Gisbourne regions in 1996.
No control programs exist in New Zealand or Australia and the disease is not notifiable.
Gross Pathology
In infected bulls, gross pathological changes are not seen. Of importance to note for collection of samples, is that T. foetus is present in all regions of the penis and prepuce with a concentration in the glans penis, and occasionally the anterior urethral orifice, but does not occur in the epididymes, ampullas, seminal vesicles, pelvic urethra or testes.
In cows, the uterus is the main organ affected. Transient infection occurs in the oviducts, cervix, uterus and vagina. The placenta is oedematous and often shows a degree of autolysis. Aborted foetuses vary in gestational age from two months to full term. Gross lesions may not be apparent but in some aborted foetuses a moderately enlarged liver is present. However, microscopic lesions occur in the placenta and foetal lungs. Trichomonads have been observed in the placental stroma, and in lung and airways of aborted foetuses and these tissues should be targeted for sample collection. A pyogranulomatous bronchopneumonia is seen, histopathologically, in many foetuses. Pregnant animals may show gross evidence of impending abortion through haemorrhagic placentomes, partial detachment of the cotyledons and placenta, and pale yellow foetal fluids containing a fine colloidal suspension. Genital tracts and foetuses may appear normal despite the infection. Pyometra is occasionally apparent and a pale yellow, semi-solid material may be present in the uterine horns, cervix and oviducts. T. foetus occurs in the uterus, oviducts, foetal fluids and placentomes and these areas should be targeted for culture.
Diagnostic Tests (General)
Diagnosis of trichomoniasis takes into account clinical history of the herd, which includes signs of early abortion, repeated returns to service and irregular oestrous cycles, and is confirmed by detection of the protozoan by culture and/or polymerase chain reaction (PCR).
Two main methods for sampling the prepuce of bulls are used; the aspiration method of Bartlett (1949) and the metal brush scraping method of Stuka and Katai (1969) as quoted by Tedesco et al., (1979). Both methods give the same diagnostic sensitivity if samples are cultured within two hours of collection. In the Bartlett method, the pipette is scraped against the glans penis and the adjacent preputial membrane before aspirating the material. Sampling from the right side of the prepuce results in a four times more likelihood of a positive result compared to a sample taken from the left side. This may be due to the position of the glans penis as the highest numbers of T. foetus are found on the portion of the glans penis excluding the galea glandis, whereas relatively low numbers occur on the remainder of the penis and prepuce. The protozoan does not reside in the lower urethra. The vagina is sampled for heifers and cows. Both collection methods are described in Part 2. Tissues from aborted foetuses are collected and samples may be inoculated into transport media in the field.
Successful growth and identification of T. foetus from samples is influenced by transport conditions and temperature. Both culture and PCR outcomes are adversely affected if temperatures exceed 37ºC for 24 hours or more during transport.47 Transport temperatures of 10-20ºC temporarily inhibit replication of T. foetus but the organism recovers once incubated at the correct temperature. Inoculated media should not be chilled or refrigerated as this will kill the protozoan. A study found that transport in phosphate buffered saline containing foetal calf serum (PBS/FCS) at 4ºC and culture within 24 hours into growth medium, or transport in PBS/FCS at 37ºC and culture into growth medium at 24 or 48 hours, was satisfactory, whereas transport at 4ºC in modified Plastridge’s medium resulted in non-viable cells. Optimum transport in Plastridge’s medium is at 37ºC. Transport at the higher temperatures requires the presence of serum (or milk). Inoculated Diamonds50 or InPouch™ TF media must be transported at 22-37ºC. Positive results are obtained at this temperature range for up to four days, whereas cultures kept at 4ºC for five days will be negative. All results are negative if samples are kept at -20ºC for three hours. As a general rule, transportation at 25ºC followed by incubation at 37ºC in the laboratory, is ideal.
A number of media have been developed for transport and culture of T. foetus; Trichomonas Medium (Oxoid), modified Plastridge’s medium, modified Diamonds medium and InPouch™ TF medium (BioMed Diagnostics). They all support the growth of T. foetus; however, with some differences that need to be taken into account when testing for T. foetus. Growth in Diamonds medium and modified Plastridge’s medium show the quickest growth with the highest concentration of cells at two to four days, compared to the InPouch™ TF medium, where the highest number of cells occurs later. Cells survive longer in the InPouch™ TF medium and modified Plastridge’s medium, as cells are present at day seven, whereas loss of viable cells occurs from day five in Diamonds medium. Culture (tested using modified Plastridge’s liver infusion medium) has a sensitivity of 72% (95% probability: 58.07-86.38%) and a specificity of 95.37% (95% probability: 94.07-96.65%) when applying Bayesian methods in the absence of a gold standard. These results are similar with the InPouch™ TF medium, which has a culture sensitivity of 67.8% and specificity of 98.8% at 95% confidence intervals for a single sample after infection of one week’s duration. Sensitivity and specificity increase to 80.0% and 98.1%, respectively, when samples are collected over three consecutive weeks.
T. foetus protozoa are visually detected in growth medium using a wet preparation examined under a light microscope or by dark ground illumination. Examination using phase contrast can assist with identification based on morphology. The identification of Tritrichomonas to species level is done using morphology after staining by Lugol’s iodine and Diff-Quick method (see Part 2 – online pdf).
Other trichomonads may be found in the normal bovine reproductive tract and need to be differentiated from T. foetus. These non-pathogenic trichomonads may be found in soil, water, urine, faeces, intestine or rumen of animals and it is important to avoid contamination from soil and faeces when collecting samples.
A number of PCRs have been reported for detection of T. foetus in bulls and cows. A real time PCR using a 3′ minor groove binder-DNA probe targeting the conserved regions of the internal transcriber spacer-1 has been developed but not submitted to SCAHLS or approved by the OIE for international trade requirements56. The probe is 2,500-fold and 250-fold more sensitive than culture followed by microscopy for smegma and mucus respectively, and detects a single cell of T. foetus per tube from smegma or vaginal mucus. It is 500-fold more sensitive than conventional PCR.
Full 25 page article is continued here:
agriculture.gov.au/sites/default/files/sitecollectiondocuments/animal/ahl/ANZSDP-Bovine-trichomoniasis.pdf
by Nicky Buller
Animal Health Laboratories
Department of Agriculture and Food Western Australia 3 Baron-Hay Court
South Perth, WA 6151
nicky.buller@agric.wa.gov.au
by Bruce Corney
Biosecurity Queensland
Department of Agriculture, Fisheries
and Forestry Brisbane, QLD
bruce.corney@daff.qld.gov.au
