A brief discussion on evaluating the effects of ruminal incubation and abomasal enzymatic digestion on the germination potential of Bromus tectorum.
https://doi.org/10.1016/j.rala.2024.05.001
The Great Basin of North America is home to a rich population of wildlife and also supports a ranching industry for rural communities, the foundation of which is supported by a mixture of important vegetative species.1,2 The disastrous introduction of cheatgrass (Bromus tectorum) to the Great Basin presents significant ecological and economic challenges.3 This invasive species has spread quickly across Western Rangelands, which has resulted in increased land management budgets as well as impacts to agricultural productivity.2,4,5
Because cheatgrass matures rapidly, it is highly competitive against native bunchgrass species.6,7 A significant hurdle for producers to overcome is that cheatgrass’s poor quality reduces its palatability as a forage for livestock.8 Considering this, the grass has led to an accumulation of fine fuel.3,9 Cheatgrass stands create favorable conditions for fire, and in turn, the fire reduces competition against cheatgrass establishment.2,10,11 This cheatgrass-fire dynamic has created a shift in the Great Basin’s natural fire cycle, resulting in the intensification of the Great Basin’s fire cycle.2,6,12
Concerns regarding livestock grazing in cheatgrass-infested rangelands has often speculated on the spreading of cheatgrass seeds through cattle excrement. To better understand if cheatgrass seeds are still viable after ruminant digestion, our study used lab-based and field-based techniques to investigate the impact that the ruminant digestive tract has on the seeds of cheatgrass.
Materials and Methods
1.0 In vitro (in glass) ruminal and abomasum incubation
Cheatgrass seeds were harvested in the fall of 2022 and in the spring of 2023 from the Greater Reno, NV area. One gram of seed was weighed and sealed into filter bags and separated based on their season of harvest. Rumen fluid was collected from two rumen-cannulated crossbred Angus steers and placed into receptacles in an incubation chamber. The seeds were incubated in the rumen fluid for 0, 6, 12, 24, 36, and 48 hours, at a constant temperature of 102.2ºF. After each incubation time, 4g of pepsin (a digestive enzyme) was added to the rumen fluid. Hydrochloric acid (HCL) was also added to make the fluid acidic (pH 3.2), this solution represented the abomasum chamber in the bovine digestive tract. The bags of seeds were incubated in this enzyme solution for 3 hours. Once this stage was complete, the bags were sorted by the season of seed collection and incubation time. Fifty seeds were counted into petri dishes in replicates of four. The seeds were counted for germination once a week for a period of five weeks. Water was added as needed.
1.2 In situ (in situation) ruminal and abomasum incubation of seeds
Five grams of cheatgrass seeds were weighed into bags. The seeds were grouped by the season of harvest. The bags were inserted into the rumen of one of four cannulated steers for incubation times of 0, 6, 12, 24, 48, and 96 hours. After the bags were removed, they were placed in an acidic enzyme solution for three hours as described in the previous section. The seeds were then placed into petri dishes in replicates of four and monitored for five weeks.
1.3 Germinating seeds in fecal material after digestion
Following the procedure for the in vitro incubation, the seeds were incubated for 48 hours, and then mixed into the feces collected from the Angus cross steers. The fecal and seed mixture had a ratio of 1:1, and was placed into tin pie dishes to form fecal pats at depths of 0.5”, 1.0”, and 1.5” in replicates of seven. The fecal pats were allowed to germinate for five weeks without water. After the first five weeks, water was added every three days for an additional five weeks.
Results
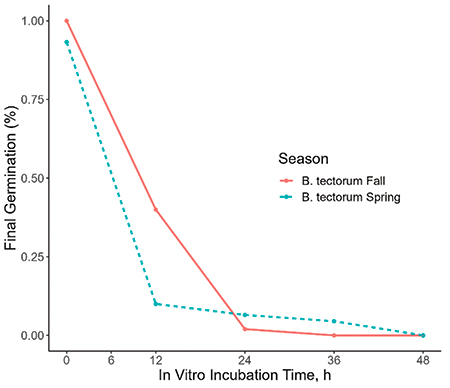
Our in vitro and in situ studies both found that the germination of cheatgrass seed decreased in association with increased ruminal incubation time. After 36 hours in the rumen, cheatgrass seed showed nearly no germination (Figure 1; Figure 2). The season in which the seeds were collected also had a significant effect, with fall harvested seeds degrading slightly slower than spring seeds (Figure 1; Figure 2). Further, our fecal germination study found that after a five-week period without water, no cheatgrass seeds germinated for any fecal depth level. Similarly, after five weeks with water allocation, no germination was exhibited amongst any fecal depth.
Conclusion
Our study highlights the negative effects that the ruminant digestive tract has on the seeds of cheatgrass and may assist in the development of targeted grazing management strategies for cheatgrass control. Results indicate that after the cheatgrass seeds are consumed by a ruminant, the seeds are sufficiently degraded by the microbes in the rumen, and are unlikely to germinate after 36 hours of being inside the rumen. While we found that there is a delay in the deterioration of seeds harvested in the fall, the consumption of these seeds will be associated with highly lignified, low quality forages which are common in the mature cheatgrass stands grazed in the fall, resulting in slower passage rates.8,13,14 Our findings offer a deeper insight into the detrimental effects ruminal conditions have on cheatgrass seeds and may ease concerns surrounding the misconception that cattle are spreading cheatgrass seeds through their excrement.
Figure 1. The evaluation of germination potential of Bromus tectorum (B. tectorum) seeds collected from the Greater Reno, NV area in October 2022 (Fall) and May 2023 (Spring) after exposure to in situ ruminal incubation (in hours) paired with 3 hours of in vitro abomasal digestion. Final germination expressed as a percentage of the total seeds examined.
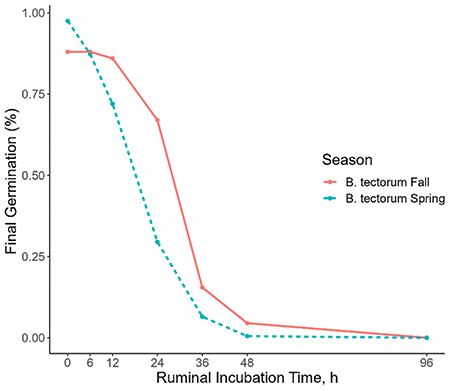
Figure 2. The evaluation of germination potential of Bromus tectorum (B. tectorum) seeds collected from the Greater Reno, NV area in October 2022 (Fall) and May 2023 (Spring) after exposure to in vitro incubation (in hours) paired with 3 hours of in vitro abomasal digestion. Final germination expressed as a percentage of the total seeds examined.
Citation
- Bailey DW, Mosley JC, Estell RE, Cibils AF, Horney M, Hendrickson JR. Synthesis Paper: Targeted Livestock Grazing: Prescription for Healthy Rangelands. Rangeland Ecology & Management. 2019; 72(6):865-877. doi:10.1016/j.rama.2019.06.003.
- Clark PE, Porter BA, Pellant M, Dyer K, Norton TP. Evaluating the Efficacy of Targeted Cattle Grazing for Fuel Break Creation and Maintenance. Rangeland Ecology & Management. 2023; 89(7):69-86. doi:10.1016/j.rama.2023.02.005.
- Clements CD, Harmon DN, Blank RR. Seed mix performance and cheatgrass suppression on arid rangelands. Rangelands. 2022; 44(2):129-135. doi:10.1016/j.rala.2022.02.003.
- Reisner MD, Grace JB, Pyke DA, Doescher PS. Conditions favouring Bromus tectorum dominance of endangered sagebrush steppe ecosystems. Journal of Applied Ecology. 2013; doi:10.1111/1365-2664.12097.
- Sheley R, Sheley J, Smith B. Cost/Benefit analysis of managing invasive annual grasses in partially invaded sagebrush steppe ecosystems. Weed Science. 2014; 62(1):38-44. doi:10.1614/WS-D-13-00056.1.
- Davies KW, Nafus AM. Exotic annual grass invasion alters fuel amounts, continuity and moisture content. International Journal of Wildland Fire. 2021; 22(3):353-358. doi:10.1071/WF11161.
- Ott, J.E., Kilkenny, F.F., Summers, D.D., & Thomson, T.W. (2019). Long-term vegetation recovery and invasive annual suppression in native and introduced postfire seeding treatments. Rangeland Ecology & Management, 72(4), 640-653. https://doi.org/10.1016/j.rama.2019.02.001.
- P Perryman BL, Schultz BW, Burrows MC, Shenkoru T, Wilker J. Fall-Grazing and Grazing-Exclusion Effects on Cheatgrass (Bromus tectorum) Seed Bank Assays in Nevada, United States. Rangeland Ecology & Management. 2020;73(3):343-347. doi:10.1016/j.rama.2020.01.012.
- Porensky LM, Baughman O, Williamson MA, Perryman BL, Madsen MD, Leger EA. Using native grass seeding and targeted spring grazing to reduce low-level Bromus tectorum invasion on the Colorado Plateau. Biological Invasions. 2021;23:705–722. doi:10.1007/s10530-020-02397-0.
- Jones RO, Chambers JC, Board DI, Johnson DW, Blank RR. The role of resource limitation in restoration of sagebrush ecosystems dominated by cheatgrass (Bromus tectorum). Ecosphere. 2015; 6(7):1-21. doi:10.3389/fevo.2019.00185.
- P Pilliod DS, Welty JL, Arkle RS. Refining the cheatgrass–fire cycle in the Great Basin: Precipitation timing and fine fuel composition predict wildfire trends. Ecology and Evolution. 2017; 7(19):8126–8151. doi:10.1002/ece3.3414.
- Williamson MA, Fleishman E, Mac Nally RC, Chambers JC, Bradley BA, Dobkin DS, et al. Fire, livestock grazing, topography, and precipitation affect occurrence and prevalence of cheatgrass (Bromus tectorum) in the central Great Basin, USA. Biol Invasions 22, 663–680 (2020). https://doi.org/10.1007/s10530-019-02120-8.
- Schmelzer, L., B. Perryman, B. Bruce, B. Schultz, K. McAdoo, G. McCuin, S. et al. Case Study: Reducing cheatgrass (Bromus tectorum L.) fuel loads using fall cattle grazing., The Professional Animal Scientist. 2014; 30:270-278. doi: 10.15232/S1080-7446(15)30112-1.
- Cook WC, Harris LE. Nutritive value of cheatgrass and crested wheatgrass on spring ranges of Utah. Journal of Range Management. 1952; 5:331–337.
